Laboratory of Genome Integrity
Research at the Laboratory of Integrative Genomics (LIG) centers on elucidating the molecular mechanisms governing DNA damage response and cellular stress pathways. These pathways are activated by a myriad of factors including, but not limited to, erroneous DNA replication, proteotoxicity, mechanical strain, and oxidative damage. Such pathways are pivotal for the sustenance of cancer cells and play a crucial role in conferring therapeutic resistance.
Our investigatory focus encompasses a wide range of cancer-specific hallmarks, such as heightened levels of DNA damage concomitant with mutational burden, challenges in DNA replication fidelity, accumulation of misfolded or dysfunctional proteins, free radical generation, nutrient scarcity, and hypoxic conditions. Through our specialized methods and domain expertise, we aim to directly interrogate and target these stress pathways within the cancer cellular milieu.
The implications of our research are manifold. They extend from the discovery of novel predictive markers to the optimization of existing anticancer regimens and the identification of innovative therapeutic targets. To achieve these aims, the laboratory is segmented into two primary research arms:
The "Cellular Stress" group, headed by Dr. Martin Mistrik, focuses on deciphering the complexities of cellular stress responses and their roles in cancer pathology.
The "DNA Replication Dynamics" group, under the direction of Dr. Pavel Moudry, specializes in exploring the intricacies of DNA replication processes, specifically in the context of malignancies.
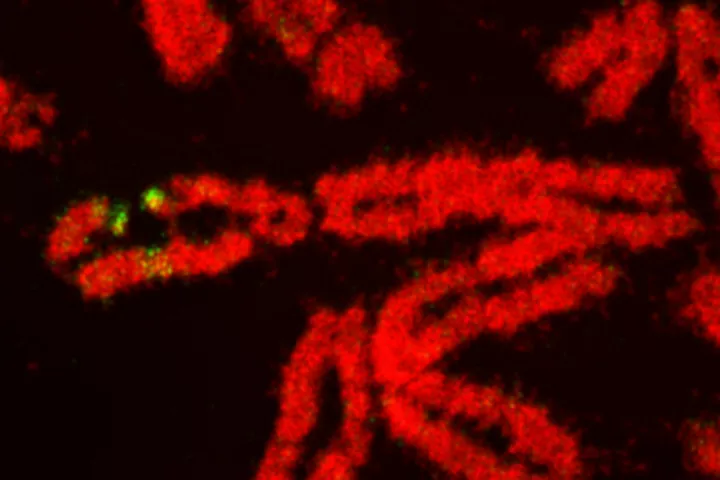
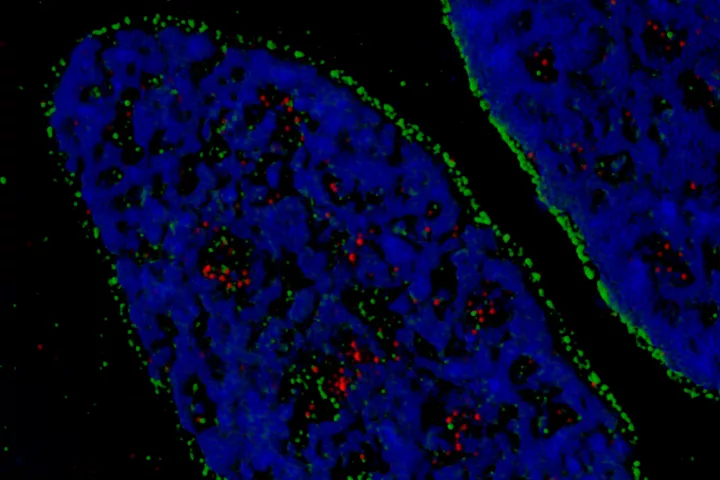
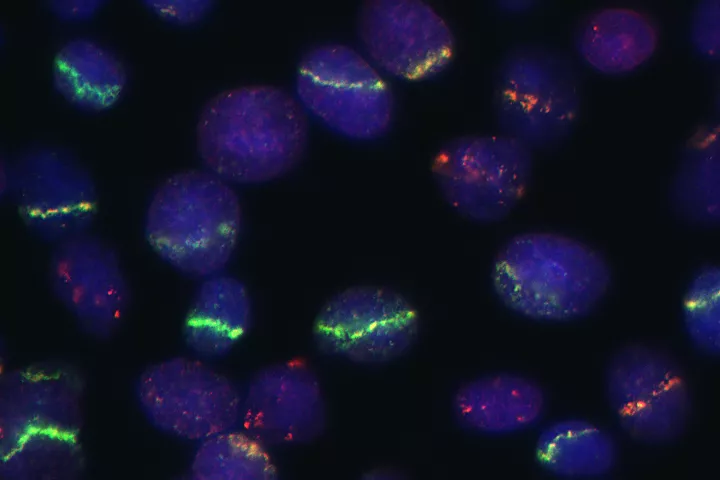
- DNA damage and repair and genomic instability
- Proteotoxic stress and proteostasis
- Mechanical stress
- Oncogenic and free radicals stress
- DNA replication and replication stress
- PARP enzyme and its targeting
- Published Application: EP 20198904.3 under EP 20198904 (7.4.2022). Ownership: Palacky University, Olomouc. Inventors: Mistrík Martin, Škrott Zdeněk, Bártek Jiří, Panáček Aleš, Kvítek Libor, Hochvaldová Lucie.
Status: Terminated
Utility Model: CZ 35118. Granted: 1.6.2021. Ownership: Palacky University, Olomouc. Inventors: Mistrík Martin, Škrott Zdeněk, Oždian Tomáš.
Status: Available
Utility Model: CZ 34546. Granted: 16.11.2020. Ownership: Palacky University, Olomouc. Inventors: Mistrík Martin, Bártek Jiří.
Status: Available
| Project: | Cellular stress in health and disease |
|---|---|
| Supervisors: | Mistrík Martin Ph.D., Moudrý Pavel Ph.D., Škrott Zdeněk Ph.D. |
| Available: | 3 |
| Intended for: | Doctoral training |
| Project: | DNA damage signaling in the cellular response to stress |
|---|---|
| Supervisors: | Mistrík Martin Ph.D. |
| Available: | 1 |
| Intended for: | Doctoral training |
| Summary: | 1 place in full-time study |
| Project: | Study of DNA double-strand break repair in tumor model |
|---|---|
| Supervisors: | Mistrík Martin Ph.D. |
| Available: | 1 |
| Intended for: | Doctoral training |
| Summary: | The so-called double-strand breaks (DSB) are the most lethal DNA lesions, which can be potent sources of mutations and thus become the beginning of irreversible genetic changes leading to degenerative illnesses, including cancer. At the same induction of DSBs introduced in the proper setup can become an unsolvable problem for the cells, particularly in specific genetic backgrounds typical for cancer, which fact is the rationale for a plethora of cancer treatment strategies. Thus the way cells deal with DSBs is under the great interest of current medicinal and biological research. The Ph.D. student will practice several methods of induction of DSBs via various sources such as gamma rays, alpha particles, UV light and various chemical inducers. Next will be studied the molecular mechanisms the cell deal with this type of DNA damage. Emphasis will be put on differences between normal and cancer cells and their potential exploration for cancer therapy. Student will also learn mutiple laboratory techniques including cell culture, RNA/DNA transfections, advanced microscopic techniques, live-cell imaging, and various biochemical analysis of proteins. |
| Project: | Signaling DNA damage in cellular response to stress |
|---|---|
| Supervisors: | Mistrík Martin Ph.D. |
| Available: | 1 |
| Intended for: | Doctoral training |
| Summary: | Our DNA is constantly under threat from DNA damaging agents. If unrepaired DNA damage can lead to errors during genome duplication, including the mutations that can lead to cancer, contribute to aging and other human diseases. Cells have evolved elaborate repair mechanisms to fix this damage and ensure that the genetic information is faithfully reproduced, and we would like to understand these repair mechanisms at the molecular level. We ultimately aim for a complete molecular and cellular understanding of critical DNA repair pathways that act on DNA damage. The Ph.D. student will practice several molecular and cellular techniques, including cell culture, RNA interference, immunofluorescence microscopy, live-cell imaging and immunoprecipitation of proteins. He/she will also learn novel and powerful techniques, such as super-resolution microscopy and purification of proteins on newly synthesized DNA. |