Lišková Barbora Ph.D.
Journals
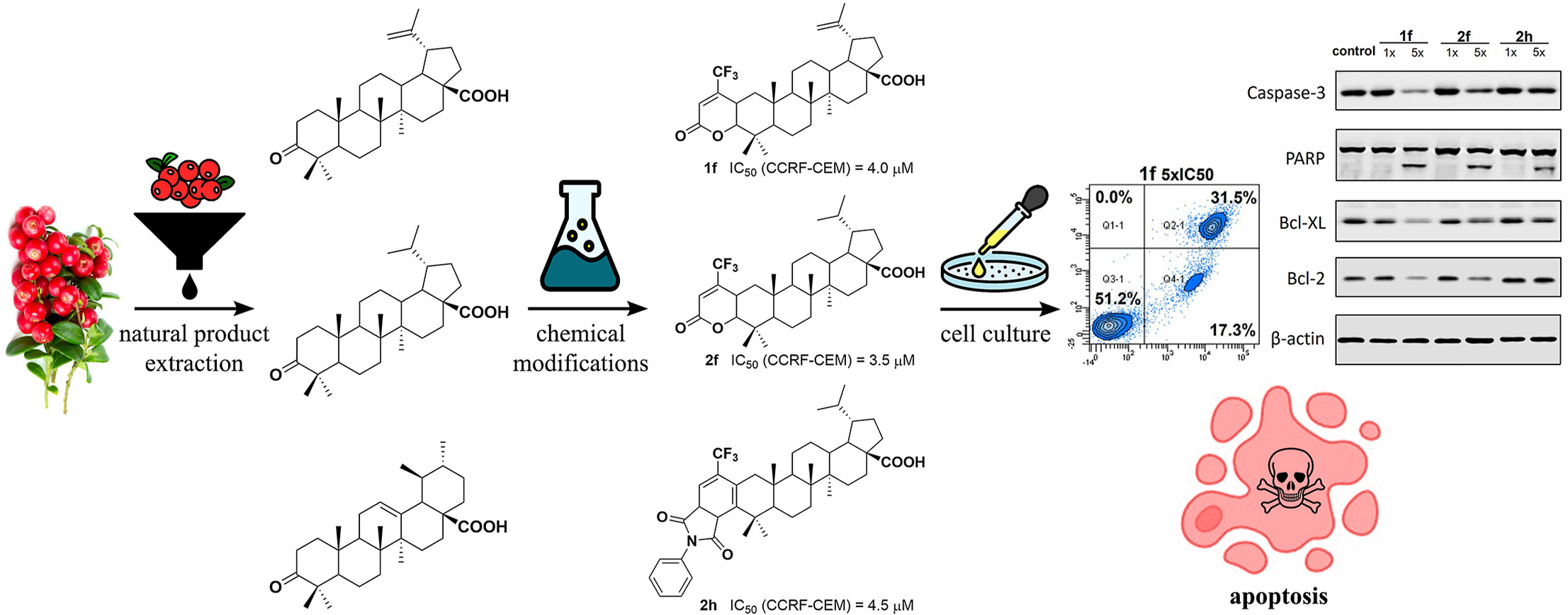
Lupane derivatives containing various aryl substituents in the position 3 have selective cytostatic effect in leukemic cancer cells including resistant phenotypes.
European Journal of Medicinal Chemistry.
2022,
244,
114850,
ISSN: 0223-5234,
PMID: 36283179,
Books & book chapters
Study of absorption, distribution, metabolism, and excretion (ADME) properties of a new drug candidates in pre-clinical development,
1.vyd.,
Olomouc,
Palacky University,
2021,
42,
325-342,
Dedication: LO1304,
ISBN: 978-80-244-6049-9,
Master mentorship
Křištofová Kateřina
ADME as support for the doscovery of new drugs in preclinical development
Status:
Ongoing from 2022.
Kašparpová Zdena
The role of the absorption, distribution, metabolism and excretion in a drug discovery in preclinical development
Status:
Ongoing from 2022.
Krupicová Kateřina
Study of the basic pharmacokinetic properties of a new drug candidates in preclinical development
Status:
Graduated from 2021 to 2023.
Bachelor mentorship
Krupicová Kateřina
Study of the basic pharmacokinetic properties of a new drug candidates in preclinical development
Status:
Graduated from 2019 to 2021.
Open positions
| Project: | Pharmacokinetic methods in preclinical drug testing |
|---|---|
| Supervisors: | Hajdúch Marián M.D., Ph.D., Lišková Barbora Ph.D. |
| Available: | 1 |
| Intended for: | Doctoral training |
| Summary: | The study of ADME properties of a potential drug that are part of the IMTM chemical library - obtained by national or international cooperation belongs to one of the first steps in predicting potential drugs. In vitro models generate many ADME parameters, including chemical, plasma and microsomal stability, plasma protein binding and proportion of passive diffusion as a transport mechanism. The Caco-2 and MDCK-MDR1 permeability assays are established models of intestinal and blood-brain barriers, respectively. Analysis of samples is performed using a Agilent RapidFire 300 - rapid online solid phase extraction with subsequent detection of the mass spectrometer Qtrap 5500 (AB Sciex) - RF/MS. High-Throughput Mass Spectrometry System RF/MS delivers ultrafast, label – free analysis of native compounds for biochemical assays in ADME and provide throughput speeds of 6 to 10 seconds per sample. |
| Project: | Study of basic pharmacokinetic properties (ADME) of new drugs in preclinical development |
|---|---|
| Supervisors: | Lišková Barbora Ph.D. |
| Available: | 1 |
| Intended for: | Doctoral training |